Ethic
After the content of the studies published in the journal, the legal responsibilities belong to the author (s) completely.
This journal,
This journal,
WMA DECLARATION OF HELSINKI ethical principles (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/);
ISMJE recommendations (https://www.icmje.org/recommendations/); (https://www.icmje.org/journals-following-the-icmje-recommendations/)
COPE (Committee on Publication Ethics/ https://publicationethics.org/guidance/Guidelines) has adopted the code of ethics.
Researches requiring Ethics Committee permission: 1. Any research carried out with qualitative or quantitative approaches that require data collection from participants using survey, interview, focus group work, observation, experiment, interview techniques, 2. Use of humans and animals (including material / data) for experimental or other scientific purposes, 3. Clinical researches on humans, 4. Researches on animals.
Issues requiring declaration: 1. Retrospective studies in accordance with the law of personal data protection, 2. “Informed consent form” in case reports, 3. Permission from the owners for the use of scale, questionnaire, photographs of others, 4. Copyright for ideas and works of art used.
TR Index Ethical Principles Flow Chart
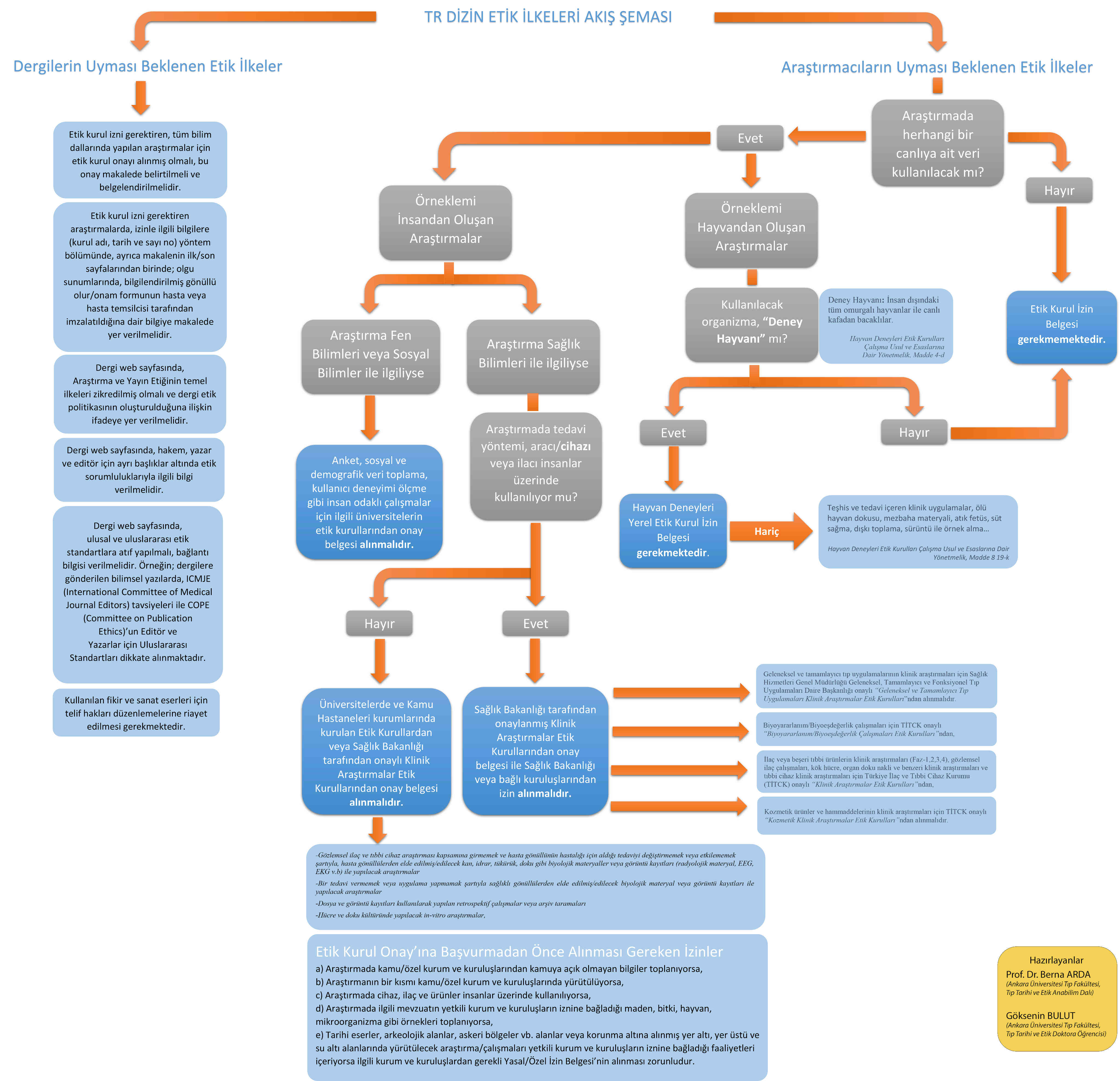
TR Index Ethical Principles Flow Chart

This content was issued on 08.04.2022 and has been viewed for 855 times.

